Create an account to track your scores
and create your own practice tests:
Test: Physical Chemistry
For constant temperature, Gibbs free energy is defined as:
Where , 


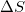
| 1. | Given that a system is spontaneous, which of the following states are possible? I. II. III. IV. |
I, II, III, and IV
I and III
IV only
I, II, and III
II and III
Physical Chemistry Tutors in Top Cities:
Atlanta Physical Chemistry Tutors, Austin Physical Chemistry Tutors, Boston Physical Chemistry Tutors, Chicago Physical Chemistry Tutors, Dallas Fort Worth Physical Chemistry Tutors, Denver Physical Chemistry Tutors, Houston Physical Chemistry Tutors, Kansas City Physical Chemistry Tutors, Los Angeles Physical Chemistry Tutors, Miami Physical Chemistry Tutors, New York City Physical Chemistry Tutors, Philadelphia Physical Chemistry Tutors, Phoenix Physical Chemistry Tutors, San Diego Physical Chemistry Tutors, San Francisco-Bay Area Physical Chemistry Tutors, Seattle Physical Chemistry Tutors, St. Louis Physical Chemistry Tutors, Tucson Physical Chemistry Tutors, Washington DC Physical Chemistry Tutors
Popular Courses & Classes
MCAT Courses & Classes in Philadelphia, ACT Courses & Classes in Boston, SSAT Courses & Classes in San Francisco-Bay Area, Spanish Courses & Classes in Chicago, SSAT Courses & Classes in Denver, ACT Courses & Classes in Atlanta, LSAT Courses & Classes in Washington DC, LSAT Courses & Classes in San Diego, ISEE Courses & Classes in Boston, GMAT Courses & Classes in San Francisco-Bay Area
Popular Test Prep
ACT Test Prep in Atlanta, MCAT Test Prep in Atlanta, ISEE Test Prep in Boston, GRE Test Prep in New York City, GRE Test Prep in Boston, MCAT Test Prep in Seattle, SAT Test Prep in Seattle, ISEE Test Prep in Seattle, GRE Test Prep in Los Angeles, LSAT Test Prep in San Francisco-Bay Area









