Acid-Base Reactions
Help Questions
AP Chemistry › Acid-Base Reactions
A buffer using acetic acid (pKa=4.76) is titrated with NaOH. What is the pH at half the equivalence point?
2.38
4.76
7.00
9.52
12.36
Explanation
The pH at half the equivalence point is equal to the pKa of the acid.
Determine the pH of a solution that is 
Explanation
Since 

Thus, ![[H_3O^+]=0.40M](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668813/gif.latex)
Recall how to find the pH of a solution:
Plug in the given hydronium ion concentration to find the pH of the given solution.
Remember to maintain the correct number of significant figures.
Put the following acids in order of their INCREASING acid strength: HI, HCl, HBr, HF.
HI, HCl, HBr, HF
HF, HCl, HBr, HI
HI, HBr, HCl, HF
HF, HBR, HI, HCl
HCl, HBr, HI, HF
Explanation
Larger halogen size leads to greater acidity because of weaker H-X interactions.
If you have a solution that consists of a monoprotic acid (HA), with a pKa of 4.1 and at a pH of 5.8, what is the predominant species present?
HA
A-
H2A+
H3O+
Equal amounts of acid and conjugate base are present.
Explanation
Since pH > pKA, the deprotonated form of the acid is predominant.
Where does the flattest region of a titration curve of the titration of a weak acid with a strong base occur?
At the pKa of the acid
At the pKb of the base
At a pH greater than 7
At a pH of 7
Explanation
In this question, titration curve would graph the pH of acid solution versus the amount of base added. Since the base is strong and the acid is weak, we can conclude that the pH will be slightly greater than 7 at the equivalence point. The equivalence point is found in the steepest region of the curve.
The half-equivalence point is the flattest region of the titration curve and is most resistant to changes in pH. This corresponds to the pKa of the acid. Within this region, adding base (changing the x-value) results in very little deviation in the pH (the y-value). This region is also the buffer region for the given acid.
Which of the following will increase the pH of an 
I. Removing carbonic acid
II. Adding sodium bicarbonate
Both I and II
I only
II only
Neither of these options
Explanation
To answer this question we need to look at the reaction below:
An increase in the pH will result in a decrease in the concentration of hydrogen ions (
![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324540/gif.latex)
Removing carbonic acid will decrease the concentration of 
![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324542/gif.latex)
Recall that salts like sodium bicarbonate, or 


![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324546/gif.latex)
Which of the following is what determines the strength of an acid?
The Ka
The Kb
Its physical state
How many bonds the central atom makes
Electronegativity values
Explanation
The Ka is the acid dissociation constant, and thus it is what determines how strong the acid is. Stronger acids dissociate to a greater extent and produce lower pH values.
Where does the flattest region of a titration curve of the titration of a weak acid with a strong base occur?
At the pKa of the acid
At the pKb of the base
At a pH greater than 7
At a pH of 7
Explanation
In this question, titration curve would graph the pH of acid solution versus the amount of base added. Since the base is strong and the acid is weak, we can conclude that the pH will be slightly greater than 7 at the equivalence point. The equivalence point is found in the steepest region of the curve.
The half-equivalence point is the flattest region of the titration curve and is most resistant to changes in pH. This corresponds to the pKa of the acid. Within this region, adding base (changing the x-value) results in very little deviation in the pH (the y-value). This region is also the buffer region for the given acid.
Put the following acids in order of their INCREASING acid strength: HI, HCl, HBr, HF.
HI, HCl, HBr, HF
HF, HCl, HBr, HI
HI, HBr, HCl, HF
HF, HBR, HI, HCl
HCl, HBr, HI, HF
Explanation
Larger halogen size leads to greater acidity because of weaker H-X interactions.
Which of the following will increase the pH of an 
I. Removing carbonic acid
II. Adding sodium bicarbonate
Both I and II
I only
II only
Neither of these options
Explanation
To answer this question we need to look at the reaction below:
An increase in the pH will result in a decrease in the concentration of hydrogen ions (
![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324540/gif.latex)
Removing carbonic acid will decrease the concentration of 
![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324542/gif.latex)
Recall that salts like sodium bicarbonate, or 


![[H_3O^+]](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/324546/gif.latex)
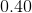



![\text{pH}=-\log([H_3O^+])](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/668814/gif.latex)

